Thailand FDA has approved Rituxikal (Rituximab Biosimilar)
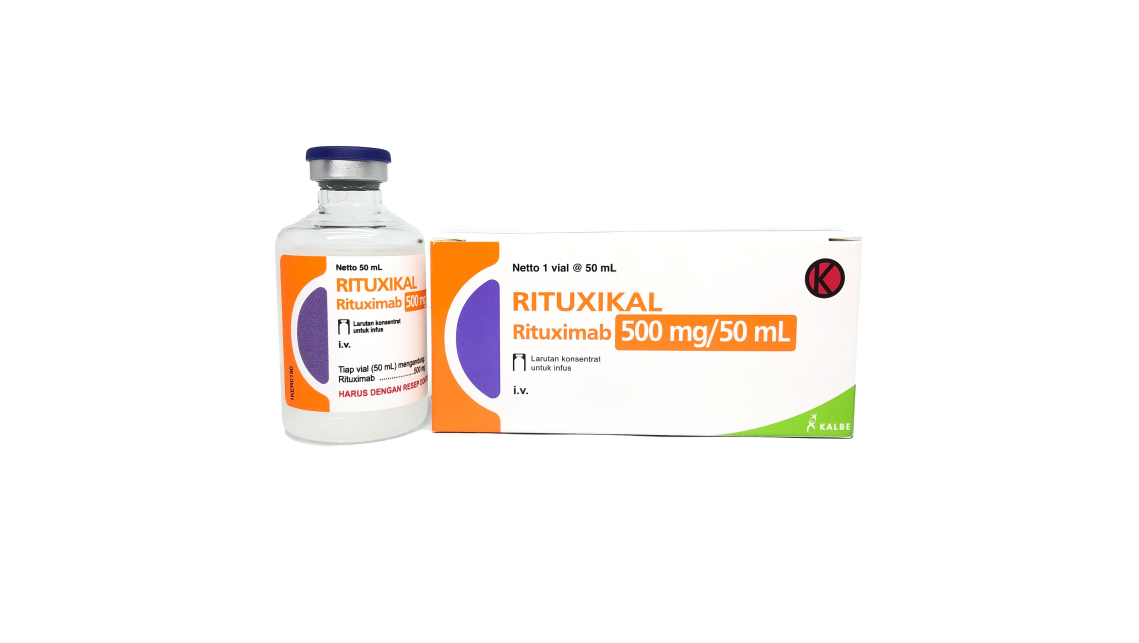
On 11 May 2022, Thailand FDA has approved Rituxikal (Rituximab Biosimilar). By this milestone, Innnogene Kalbiotech spread the wing not only to local market, but also to South East Asia market. Rituxikal has 2 strengths: Rituxikal 100 mg / 10 mL and Rituxikal 500 mg / 50 mL. Rituxikal is currently undergo technology transfer process to be produced in PT Kalbio Global Medika, Cikarang, Indonesia. PT Kalbio Global Medika (KGM) is a contract GMP biomanufacturing company specialized in the use of mammalian cells. KGM is also a part of Kalbe Group and subsidiary company of PT Kalbe Genexine Biologics (KGbio).
